What Are the Final Partial Pressures of H2 and N2
For each of the following. Processes Ideal Gas A steady flow compressor handles 1133 m 3 min of nitrogen M 28.

For The Reaction N 2 G 3h 2 G Harr 2nh 3 G The Partial Pressure Of N 2 And H 2 Are 0 80 And 0 40 Atmosphere Respectively At Equilibrium The Total Pressure Of The System Is 2 80 Atm What Is
The final percentage of NO mole fraction is about 14.

. The final temperature of the reaction reaches about 2720 K. Decreasing the H2 d. The changes in KE and PE are negligible.
The gas is a bubble so the pressure remains 10 atm but the volume will vary as the gas reacts with the water. Initially the partial pressures of dry air CO2 N2 O2 and H2O--10-35 078 2 and 0 atm. TK PH2 PI2 PHI K 298 00302 00388 0922.
Both a and b e. Increasing the temperature b. H2gI2g2HIg Complete the table.
The concentration of partial species and combustion temperature variations in D50 combustion. K 1399 measured at intake where P1 97 KPa and T1 27 C. Assume that all partial pressures are equilibrium values and in bar.
None of these effect rate. The problem is defined as follows. Pure water reacts with a gas volume that is initially 10 L 25C and pressure 10.
Download high-res image 150KB Download. Discharge is at 311 KPa. For the reaction N2 3H2 -- 2NH3 The rate law is Rate kN2H2Which of the following will decrease the rate of the reaction.

Ammonia Synthesis From N2 And H2 A Correlation Of Ammonia Yield With Download Scientific Diagram
The Pressure Of A Mixture Of H2 And N2 In A Container Is 1200 Torr Sarthaks Econnect Largest Online Education Community
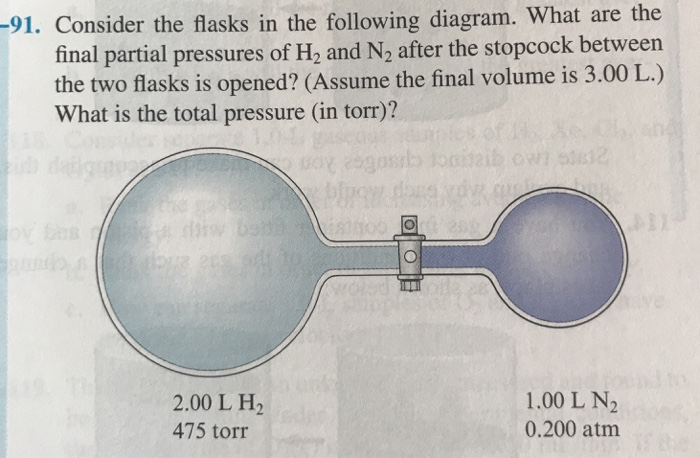
Solved 91 Consider The Flasks In The Following Diagram Chegg Com
No comments for "What Are the Final Partial Pressures of H2 and N2"
Post a Comment